Manufacturing of cell products

Your CDMO partner for cell and gene therapy products
Cellex is unique! As a full-service provider we can offer comprehensive expertise in all areas of cell therapy manufacturing – from providing cellular starting material to manufacturing and delivery of finished cell therapy products. We can support you from preclinical development through clinical manufacturing to commercialization.
Why choose Cellex for cell and gene therapy manufacturing?
Expertise:
We have more than 10 years’ experience in the manufacturing and testing of intermediate and final cell and gene therapy products.
Production capacity and flexibility:
Cellex’ manufacturing facilities offer substantial capacity. We have a high level of expertise in equipping cleanrooms to customer specifications and rooms can be designed individually.
Product quality:
We have a broad portfolio of analytical technologies, applied in state-of-the-art quality control laboratories. Taking into account all existing laws and guidelines, we can ensure controlled, compliant, and consistent high-quality production.
Timeline:
As a full-service provider with expertise in all areas we support you in developing products as quickly as possible and ensuring a smooth process. A dedicated program manager will develop and organize a project plan and timelines.
Process development and optimization:
We can support you from the technology transfer of your research product through process optimization to the commercial production of your ATMPs/CGTs. You will also benefit from our experience in commercial process optimization, which can lead to significant cost savings.
QP services:
Batch certification is carried out by a team of highly experienced Qualified Persons (QPs).
Logistics:
Cellex provides customized and GDP-compliant transportation options. The geographical location of the production facilities in the middle of Central Europe assures logistical advantages through established transport networks.
Trained employees:
Intensive training in our in-house training center guarantees that our specialized and experienced operators are always completely up to date.
Project management:
Throughout the entire project cycle by highly qualified and experienced project managers. Program managers support the development, implementation, optimization and qualification of processes.

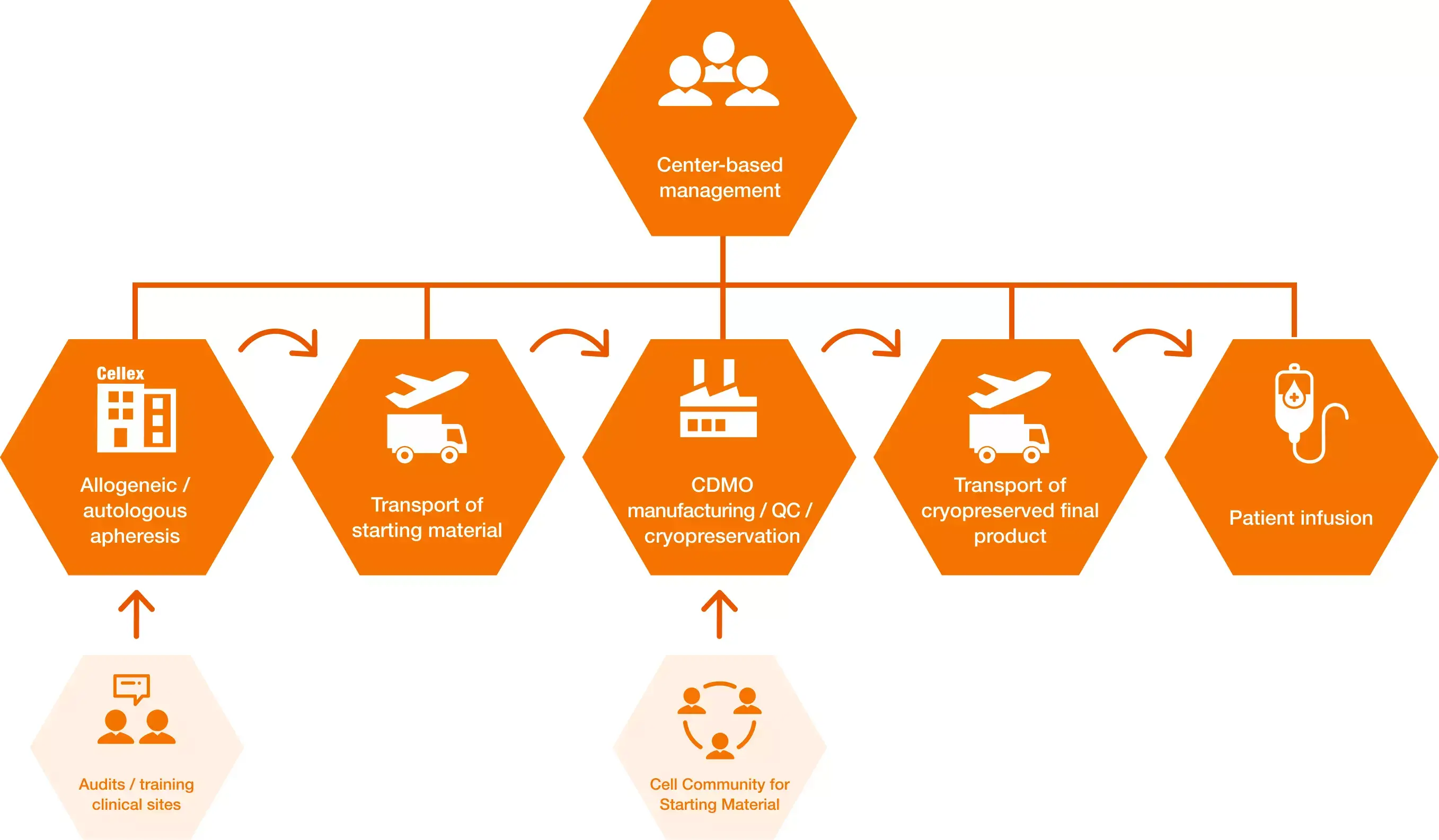
From strategy to market launch
Complex development and production process for cell-based therapeutics
In our modern Cellex Cell Manufacturing Plant (CCMP), we manufacture both innovative intermediate cellular products (ITM) and final drug products (FDP), thus supporting our customers in their goal of providing patients who suffer from a wide range of diseases with access to new therapies. Production is based on allogeneic or autologous cell material and is performed in accordance with customer specifications and GMP guidelines.
Our highly qualified employees will support you with their expertise in individual process development. With the manufacturing license in hand the products can be used in clinical trials and for commercial purposes. If you already have a defined manufacturing process, we will carry out a technology transfer and handle the process development to large-scale manufacturing.
Currently manufacturing 4 FDPs (final drug products) and 7 cellular intermediates

27,000 sq. ft. GMP-approved production facility incl. clean room suites and ballrooms

5,800+ batches produced; 100% of the batches requested by the sponsor were successfully manufactured

Manufacturing of ATMPs/CGT products
The GMP-compliant facility has state-of-the-art class C and D clean rooms with individually adaptable and equipped production suites. Over the years, we have gained extensive expertise in transferring preexisting manufacturing processes into a closed system approach by using sterile docking. If open process steps (Class A/ISO 5 containment) are required, these are performed in an isolator.
The following standard process steps are established and used for multiple clients:
- Selection or depletion of specific cell populations
- Genetic modification of cells, e.g. using viral vectors or CRISPR/Cas9 technology
- Expansion
- Fill & finish
- Cryopreservation
Contact us to discuss how we can support you in the production of ATMPs/CGT products and how you can benefit from our experience.
Quality management
Cellex works with a pharmaceutical quality system to ensure compliance with all relevant laws and regulations, including:
- EudraLex Volume 4, in particular Part IV, Annex 1, 11, 13, 14, 15, 16, 19
- ICH Q2, Q5C, Q6B, Q7, Q8, Q9, and Q10
- Guideline on quality, non-clinical and clinical aspects of medicinal products containing genetically modified cells (CAT/CHMP/GTWP/671639/2008)
- Guideline on human cell-based medicinal products (EMEA/CHMP/410869/2006)
- Guideline on potency testing of cell-based immunotherapy medicinal products for the treatment of cancer (EMEA/CHMP/BWP/271475/2006)
Our team ensures that relevant processes, such as change control, corrective and preventive action (CAPA), and deviation management, run smoothly.
The entire production process is monitored from the first qualification steps of the devices and procedures to the release of the product. Associated documents, such as SOPs, qualification and validation protocols, material and supplier qualification, etc., are created and maintained by the quality management staff. Our quality management system (QMS) is constantly monitored and updated to ensure compliance with statutory regulations and with the highest quality and safety standards.
If you would like to perform audits, this is possible by prior arrangement.
Quality control
Quality Control with a broad spectrum of methods is performed in-house or at contracted external laboratories using validated methods.
Analysis of various cell populations through determination of size, granularity and antigen expression on the cell surface using fluorescent-labelled antibodies
Automatic quantification and analysis of cells using impedance measurement and imaging cytometry
Quantitative measurement of biological activity
Our employees also have comprehensive expertise in cryopreservation and will advise you on the cell transport. Read more here about cell transport.
QP release
An experienced team of Qualified Persons (QP) is responsible for compliance with the relevant pharmaceutical regulations. They review and check all relevant data and documentation, and they certify the product against an IMPD or marketing authorization.